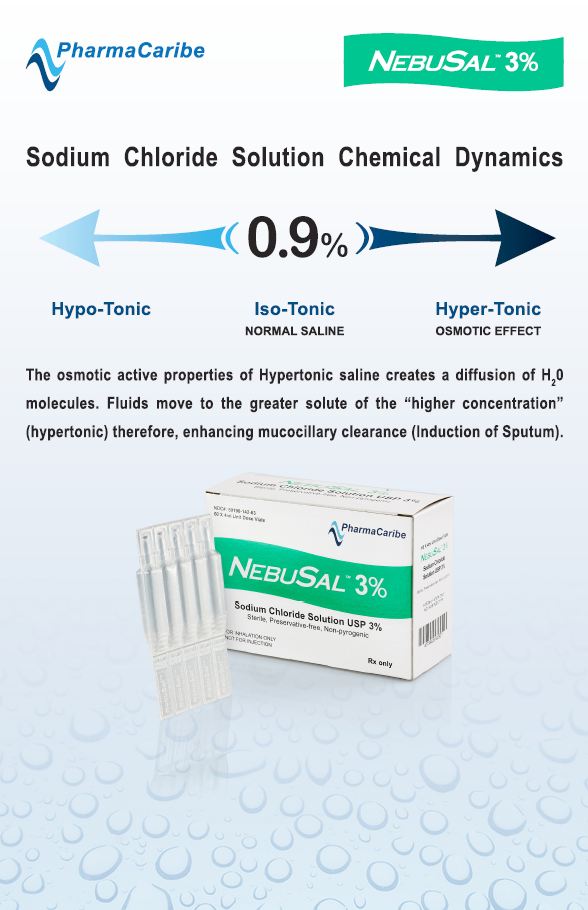
Welcome to PharmaCaribe.com – makers of PulmoSal, NebuSal, and other hypertonic saline inhalation solutions. Today PharmaCaribe is a global leader in saline inhalation solutions with several products designed strictly to USP standards and a line of (pH+) Bio-Balanced™ formulations FDA cleared for commerce. Read more below.


HYPERTONIC SALINE SOLUTION INHIBITS SARS-COV-2 REPLICATION IN VITRO
A recent Brazilian study by Machado et.al presented data in a not yet peer certified reviewed preprint report that suggested hypertonic saline solution (HS) may have a possible role as a prophylactic and as an alternative therapeutic option for COVID-19.
In vitro, the potential antiviral activity of sodium chloride (NaCl) has been already studied for RNA viruses such as mengovirus13, respiratory syncytial virus (RSV) and others. Furthermore, a randomized controlled clinical trial studying the effectiveness of treatment of viral upper respiratory tract infection, using hypertonic saline nasal irrigation and gargle (HSNIG)15, observed a decrease in the duration of illness, over-the-counter medication use, transmission within household contacts and viral shedding15.
Furthermore, findings from a post-hoc analysis of NCT02438579 clinical trial suggest that hypertonic saline nasal irrigation and gargling may play a role in reducing symptoms and duration of illness caused by COVID-1949.
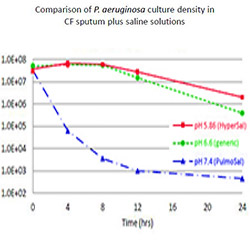
The experiments were performed to test if hypertonic saline solution is able to inhibit SARS-CoV-2 virus replication in vitro.
The SARS-CoV-2 inhibition assays were performed using monkey kidney Vero cells and as lung epithelial cells, may be heavily infected by SARS-CoV-216 and are capable of undergoing endo- and exocytosis, being therefore a good model cell to study the SARS-CoV-2 life cycle.

FEATURED LINK